Research
We study various research themes in synthetic molecular inorganic and organometallic chemistry and we aim to discover fundamentally new compound classes including those with elements in rare oxidation states, unusually bonded fragments and rare and reactive metal hydride species. These generally show novel structures, have unseen properties and as a consequence often show a unique reactivity. We use these for applications in chemical synthesis, catalysis and other technologies. In our studies, we use complexes with elements from across the periodic table with a strong focus on main group elements.
Many main group elements are earth abundant, non-toxic and even biocompatible. For example, the eight most abundant elements in earth’s crust account for approximately 98-99% of its mass, and seven of these are main group elements. Between them, they offer a wide range of properties, ionic or covalent radii, and accessible oxidation states. In our research, we employ and design a wide range of sterically demanding neutral and anionic ligands. We constantly try to push the boundaries of what is synthetically achievable. For manipulations of air and moisture sensitive compounds, we routinely apply specialized synthetic Schlenk line and glove box techniques in our modern laboratories at the University of St Andrews. The main characterization methods we use to study these systems include multi-nuclear NMR spectroscopy and single crystal X-ray diffraction techniques among numerous other methods.
Unusual main group molecules and their reactivity: low oxidation state species, donor-stabilized small molecules and compounds with rare bonding modes
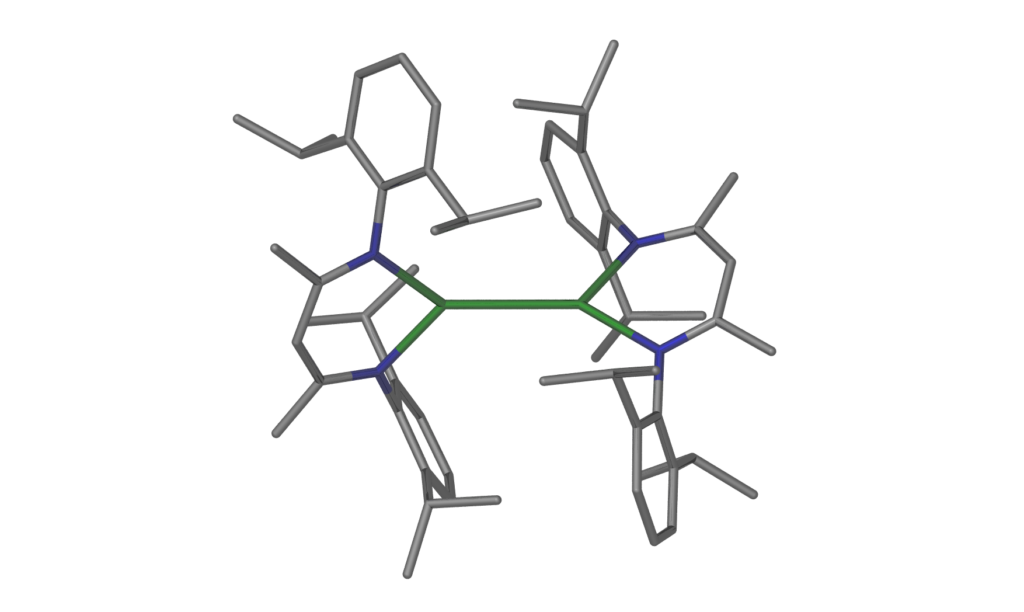
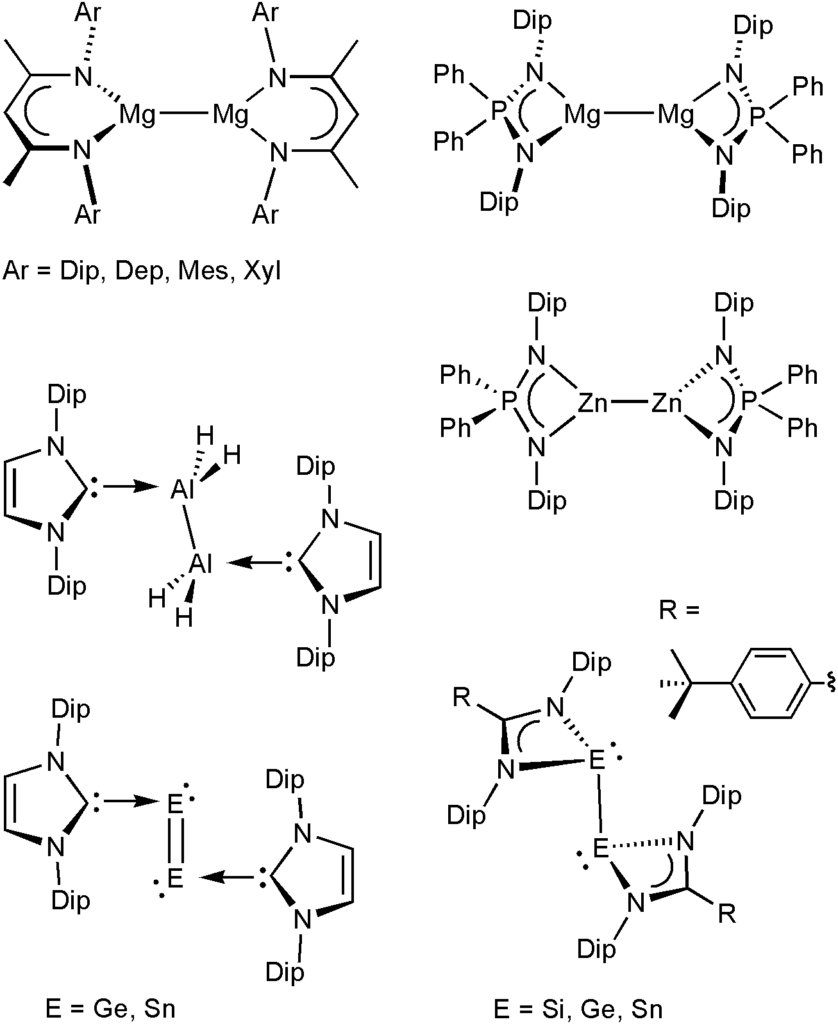
We study the chemistry of unusual molecules of the s- and p-block elements including rare oxidation states and elements in novel bonding situations. Selected examples and reaction products can be found in the pictures. For example, we have previously prepared the first stable magnesium(I) compounds with the general formula LMgMgL (in collaboration with the Jones group). These compounds contain a central Mg22+ core with a high s-character covalent Mg–Mg bond that is coordinated by anionic ligands. We study the chemistry of these compounds towards a range of organic, organometallic and inorganic substrates, including small molecules and gases, and develop new stoichiometric reactions and catalytic systems. These easy to synthesize and ‘bottleable’ reagents have furthermore become invaluable tools as strong, selective, stoichiometric, hydrocarbon-soluble, and safe reducing agents in the reduction chemistry of inorganic molecules and organometallic complexes. In many cases, the new molecules could only be synthesized only when a dimeric magnesium(I) compound was employed as the reducing agent. For example, we were able to isolate unusual donor stabilised digermanium(0), ditin(0), and dialane(4) (Al2H4) molecules stabilized by N-heterocyclic carbenes, and a range of stable compounds of univalent elements. More generally, we are studying the chemistry of various unusual compound classes with a range of sterically demanding ligands from groups 1, 2 and 12-15 of the periodic table.
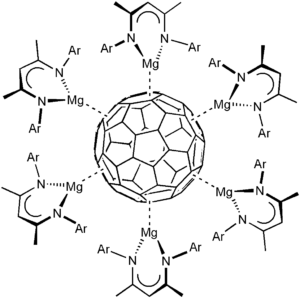
Dimeric magnesium(I) complexes react readily with fullerene C60 to a series of contact ion complexes [(LMg)nC60] (n = 2, 4, 6) with central C60n- ions and ionic magnesium-fulleride interactions, see the image for an example of C606-. The facile reduction of C60 also highlights the very strongly reducing character of LMgMgL compounds and gives an estimate of their reduction potential (at least ca. E = -2.9 V vs SCE).
Main group hydrides: soluble s-block metal hydride complexes, well-defined cluster compounds, unusual p-block hydrides and new reactivity
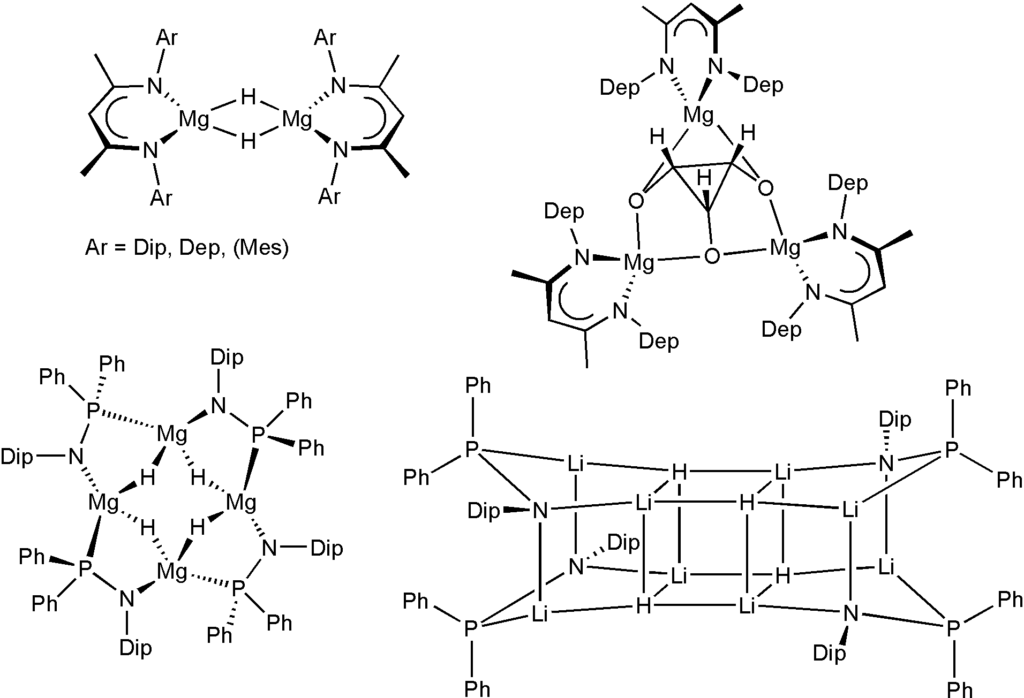
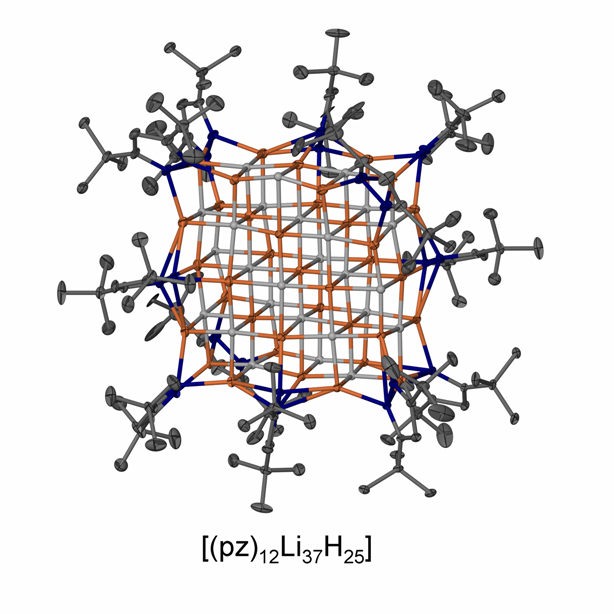
We have an interest in the synthesis of novel molecular main group element hydride species, their properties, structures, and their stoichiometric and catalytic reactivity. This includes the chemistry of rare s-block metal hydride complexes, p-block metal hydride complexes and reactive hydrogen derivatives of p-block elements in normal or high oxidation states. Redox reactions and interconversions of main group element species with concomitant hydrogen loss, transfer, and uptake are of interest including their potential in novel catalytic transformations and for hydrogen storage solutions. Soluble, well-defined metal hydride complexes of the s-block metals are rare despite their potential importance for synthesis, catalysis and hydrogen storage in part because of the ionic nature of metal-ligand interactions. Now, synthetic routes and suitable stabilizing ligand systems have been identified that allow the study of these systems. These include for example the neutral magnesium hydride complexes with b-diketiminate ligands and phosphinoamide ligands, and a hydrocarbon-soluble lithium hydride complex with central (LiH)4 cube. We could furthermore extend this strategy to afford up to nanometer-sized, hydride-rich LiH clusters stabilized by simple pyrazolate ligands that could be structurally characterized, e.g. see picture, and the first NaH complex [(pz)6Na7H] (pz = 3,5-di-tert-butyl pyrazolate). These molecular complexes show a much higher reactivity compared with insoluble bulk metal hydrides and can, for example, undergo rapid hydrometallation reactions of organic substrates including a unique cyclopropanetriolate complex. Some species are furthermore highly active catalysts for the hydroboration and hydrosilylation of ketones at very low catalyst loadings, and other transformations.
Ligand design
The steric and electronic profiles of ligand systems are crucial for the stabilization of novel species and their further reactivity. To obtain novel compound classes we employ, design and develop ligand systems that promise access to and stabilization of our target systems, aid with reaction monitoring, separation and compound characterization. Often, small changes to the steric or electronic profile of a ligand can lead to fundamentally different outcomes for a given system. In the past we have used a large range of neutral and anionic ligand systems incl. amines, imines, N-heterocyclic carbenes and related species, phosphines, alkyls, aryls, alkoxides, amides, amidinates, guanidinates, b-diketiminates, pyrazolates, phosphinoamides, iminophosphoranes, substituted methanides- and methandiides, cyclopentadienides and related species, and constantly reassess what is beneficial for our chemistry.